In partnership with Pittsburgh Post-Gazette.
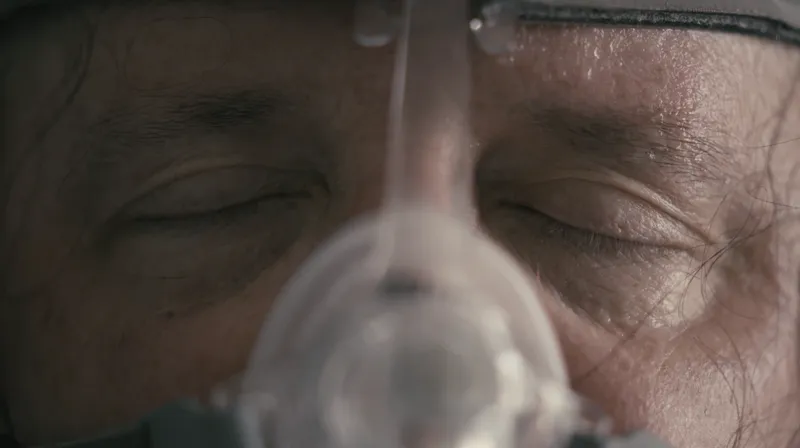
The massive recall of DreamStation machines and other breathing devices disrupted medical care for millions in the United States and around the world. About 20 models of ventilators, CPAP (continuous positive airway pressure) machines and BiPAP (bi-level positive airway pressure) machines were affected. The U.S. government has classified the recall as the most serious type, one for device defects that can cause serious injury or death.
An investigation by ProPublica and the Pittsburgh Post-Gazette found that the company continued to sell the devices long after it discovered that foam inside them could break down in heat and humidity, sending material that could have “toxic and carcinogenic effects” into the noses, mouths, throats and lungs of users. The reporting team collaborated with Mediahuis NRC, the publisher of one of the largest newspapers in the Netherlands, where Philips’ parent company is located.
In a series of statements, Philips said it acted as soon as it learned of the “potential significance” of the problem.
But an investigation by the newsrooms of the 11 years between the first complaints and the recall reveals a different story — one of a company that sought to protect its marquee products as stock prices soared. Again and again, previously undisclosed records and interviews with company insiders show, Philips failed to share, with regulators or the public, the mounting evidence that its highly profitable breathing machines threatened the health of the people relying on them, in some cases to stay alive.
The company has said testing so far on its machines shows they are unlikely to cause “appreciable harm.” But experts say the test results are concerning and the Food and Drug Administration has said that the machines present an “unreasonable risk to patients.”
In this virtual conversation, we sort through the facts of the investigation and the company’s response. Register below and submit your questions to our expert panelists.
Speakers include:
- Debbie Cenziper, ProPublica reporter
- Michael Sallah, Pittsburgh Post-Gazette deputy managing editor of investigations
- Muhammad A Rishi, MBBS, Chair Public Safety Committee, American Academy of Sleep Medicine
- Radhika S. Breaden MD, MPH, DABMS Sleep Medicine
- Rita F. Redberg, MD, MS, Professor of Medicine at UCSF and cardiologist
- Teresa Lindeman, Pittsburgh Post-Gazette managing editor of news and features
- Ziva Branstetter, ProPublica senior editor
Help ProPublica and the Pittsburgh Post-Gazette investigate the recall of Philips Respironics breathing machines. Share your experience with us.
This event has ended.